FUEL & FUEL SYSTEM MICROBIOLOGY PART 18 – PREVENTING MICROBIAL DAMAGE TO FUEL SYSTEMS PART 1
Disclaimer:
I’ll open this post with a disclaimer. Microbes are ubiquitous. There are extraordinarily few habitats on earth where thriving, microbial communities have not been detected. In practical terms, this means that it is unlikely that operators will ever have a completely sterile fuel system or that they will reduce their fuel system biodeterioration risk to zero. Biodeterioration can still occur in the best maintained fuel systems. However, the risk of it occurring in an inadequately maintained system is much more likely.
Biodeterioration:
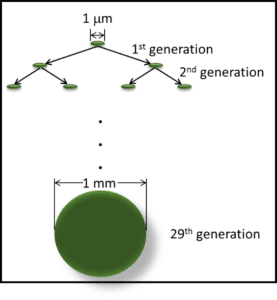
I’ll also take this opportunity to remind readers that biodeterioration (damage caused by organisms) and bioremediation (using microbes or other organisms to degrade or remove toxic or noxious chemicals) are the flip-sides of the biodegradation coin (figure 1).
For now, I’ll focus on fuel system biodeterioration. Most of the damage caused to fuel system components is caused by biofilm communities (see post #16 https://biodeterioration-control.com/2017/11/). Microbes cause damage either directly or indirectly (more on this in a future post). The most obvious indications of biodeterioration are filter plugging and corrosion. Although it’s typically the first indication of a biodeterioration problem, filter plugging is a late symptom. I often compare it to a heart attack; a late – but often first recognized – symptom of coronary disease.
So how do we substantially reduce fuel system biodeterioration risk? Step one is cost-effective condition monitoring (CM). Step two is cost-effective predictive maintenance (PdM; see https://biodeterioration-control.com/microbial-damage-fuel-systems-hard-detect-part-5-predictive-maintenance-pdm/). The former drives the latter. Why do I emphasize cost-effectiveness? As I see it, there is as little justification for investing $10,000 per year to detect problems that might cause $1,000 per year of damage, as there is in refraining from spending $10,000 per year to detect problems that could cost $100,000 per year. I’m not suggesting that any fuel system CM program should cost $10,000 per year. An effective program can cost less than $2,000 per year. My point here is that before setting up a CM/PdM program, stakeholders should invest a bit of time and effort to determine their actual annual biodeterioration-related costs.
Opportunity Cost:
Nearly two decades ago, I first argued that a 10% flow-rate loss at high-traffic, retail sites, can easily translate to more than $100,000 per dispenser per year opportunity cost (Passman, F.J., 1999. “Microbes and Fuel Retailing: The Hidden Costs of Quality.” Nat. Petrol. News 91 [7]: pp: 20-23). My model did not include lost C-store revenues related to customer discontent with their fueling experience. Retailers who have tested my model have invariably been shocked by the huge impact of seemingly minor flow-rate reductions on fuel sales volumes. I’m still trying to understand the psychology behind retailers’ general reluctance to even test my model (for model details, contact me at fredp@biodeterioration-control.com). Bottom line: the return on investment (ROI) for well-designed and executed, CM can easily be >$1,000 return on each $1 invested.
Condition Monitoring:
In Parts 2 through 18 of this series, I’ve written about the details of condition monitoring. I won’t repeat that information here. Instead, I’ll offer a few basic guidelines:
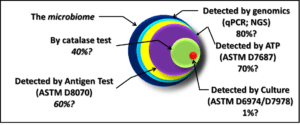
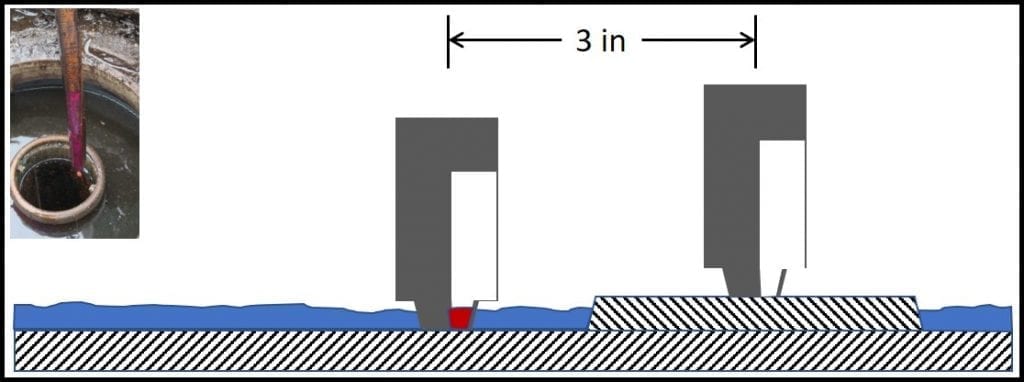
1. Testing hierarchy – CM plans should include two or more tiers. Tests that are easiest and least expensive to perform should be done most frequently (checking UST for bottoms-water accumulation and dispenser flow-rate checks are great examples of Tier 1 tests). Tier 2 tests include bottom-sample visual inspection (optimally, samples should be collected from the fill, automatic tank gauge, and submerged turbine ports). A simple microbiological test (for example ASTM D7687) is indicated whenever the bottom-sample is turbid or when it includes water. When Tier 2 tests indicate that the biodeterioration risk is moderate to high, Tier 3 tests (generally performed by a qualified laboratory) are used to confirm the risk.
2. Test method selection – notwithstanding the examples I mentioned under Testing hierarchy each site owner should develop a CM plan that best meets their needs. You can read my test specific blog posts (or ASTM D6469) for discussions of the benefits and limitations of each test method.
3. Testing frequency – my rule of thumb, after you have determined how often a test parameter is likely to indicate an increased biodeterioration risk, divide that period in three. That gives you the optimal test interval. Testing more often typically translates into greater costs without any real ROI. Testing less frequently increases the risk of having to perform corrective – rather than preventive – maintenance actions.
4. Understanding trends – each of the three previous guidelines depends on a basic understanding of trends. For example, even fuel with an ISO 4406 cleanliness rating of 18/16/13 (see https://www.iso.org/standard/72618.html), will eventually plug dispenser filters. Consequently, dispenser flow-rates will invariably decrease. How many operators know what normal looks like? How many have a control limit? Typically, dispenser filters can process >250,000 gal of fuel before the flow rate will fall below 7 gpm. Replacing fuel filters when the flow-rate is <7 gpm strikes a balance between the opportunity maintenance costs. Knowing whether the flow rate has fallen to <7 gpm after 50,000 gal or 500,000 gal of fuel have been filtered serves to trigger additional sampling and testing. If the amount of fuel filtered before substantial flow reduction occurs is much less than expected, then additional testing is indicated.
Summary:
In summary, microbial contamination control depends on a good CM program that is linked to a good PdM program. A reasonable investment in CM and PdM should be a fraction of the likely cost impact of not having those programs in place. A cost-effective CM program is driven by an understanding of system trends, definition of the methods that will provide the most useful, actionable information; selection of which tests to run; and determination of sampling and testing frequency. In my next blog, I’ll focus on preventive measures. In the meantime, if you have questions or comments about today’s post, please contact me at fredp@biodeterioration-control.com.